Introduction:
Clonal hematopoiesis with indeterminate potential (CHIP) constitutes a part of the biological aging process and comprises the acquisition of somatic mutations in hematopoietic stem cells (HSCs). In the context of allogeneic hematopoietic stem cell transplantation (HCT), the potential transfer of CHIP from donor to recipient may raise concerns on HCT-related complications. There are contradictory data on donor CHIP's impact on long-term transplant outcomes using very diverse conditioning regimen as well as various graft-versus-host disease (GVHD) prophylaxis. This study aims to evaluate the impact of donor CHIP on allogeneic HCT outcomes in a cohort of the patients transplanted using a uniform, myeloablative intensity conditioning of Fludarabine-Busulfan (4 days) as well as Cyclosporine/Methotrexate with 4.5 mg/kg rabbit antithymocyte globulin (ATG) over 3 days for GVHD prophylaxis.
Patients and methods:
Peripheral blood samples were obtained from recipients and their corresponding donors who underwent allogeneic HCT at Tom Baker Cancer Centre, Calgary, Canada. Samples were archived from 339 patients and 255 donors prior to HCT, and additional patient sampling was done on day 28 (D28), day 56 (D56), or day 84 post allogeneic HCT (D84). As a result, paired recipient-donor samples were available for 255 HCTs. This study was approved by the institutional review boards of Princess Margaret Cancer Centre, Toronto, and Tom Baker Cancer Centre, Calgary. For the detection of CHIP, we have applied a bar-coded error-corrected sequencing protocol, a single-molecule molecular inversion probe capture protocol (NAR Genom Bioinform, 2022), which was performed at Wiesmann Institute of Science (Rehovot, Israel). We designed probes targeting 34 genes covering CHIP mutations along with other AML related mutations. Bar-coded NGS library was generated and processed. All statistical analyses were performed using R statistical software 3.5.0 and EZR version 1.41.
Results:
Sixty-seven clonal mutations in 56 recipients (18.2%) and 22 clonal mutations in 18 donors (7.1%) were detected prior to HCT. For recipients, TET2, JAK2, RUNX1, PPM1D, and DNMT3A were detected in at least 2 patients or more. For donors, the most frequently mutated gene was TET2 (3.1%), and the seconds were DNMT3A and PTPN11 (1.2% for both).
Baseline characteristics of 255 patient-donor pairs did not show any difference between D-CHIP and no D-CHIP groups except donor age. Donor CHIP status was distributed evenly throughout most of the demographics of recipients, type of disease, pre-transplant status, and transplant procedure. Most of the pts received peripheral blood stem cells (97%).
With a median follow up duration 6.02 years, the 5-year OS rates tend to be higher in no D-CHIP group than D-CHIP group (63.1% vs 42.8%) with a HR of 1.73 (95% CI [0.90-3.35]), although it was not statistically significant (p=0.096). The cumulative incidence of relapse for both groups were not different in 5 years (28.1% vs. 28.4%, p=0.849). The cumulative incidence of NRM within 5 years in D-CHIP group was slightly higher than no D-CHIP group (28.4% vs 17.5%), but it was not statistically significant (p=0.250). Neutrophil engraftment by day 28 was 99.1% vs. 100%, without statistical difference between the two groups (p=0.785).
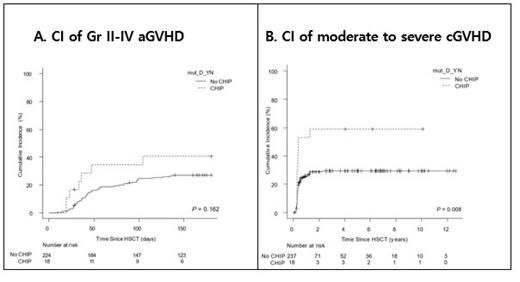
The cumulative incidence of grade II-IV acute GVHD within day+100 seemed to be higher in D-CHIP group compared to no D-CHIP group, but it was not statistically significant (24.8% vs. 34.5%, p=0.148; Figure A). In terms of chronic GVHD, cumulative incidence of moderate to severe GVHD was significantly higher in 5 years after transplant (29.4% vs. 58.8%; HR 2.55 [1.331-4.96], p=0.006; Figure B). Even when we set the cut off level of donor CHIP positivity up to >1% or >10%, the association between donor CHIP and risk of chronic GVHD remained significant.
The impact of donor CHIP on the cumulative incidence of moderate to severe GVHD was confirmed in multivariate analysis (HR 2.3 [1.3-4.1], p=0.003). Other factors associated with moderate to severe chronic GVHD were previous history of grade II-IV acute GVHD (p=0.008) and donor type (p=0.054).
Conclusion:
Under clinical setting of uniform conditioning regimen and GVHD prophylaxis incorporating ATG (4.5mg/kg), donor CHIP was found to be associated with higher risk of moderate to severe chronic GVHD, while it does not affect other allogeneic HCT outcomes.
Disclosures
Storek:Sanofi-Pasteur: Research Funding. Kim:Novartis: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Pfizer: Honoraria, Research Funding; Paladin: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal